Abstract
Introduction It has been made great commercial success in hematological malignancies by chimeric antigen receptor (CAR) T cell therapy. However, high rate of adverse events (AEs) still remains an issue. Non-viral Genome Targeting CAR (gtCAR) delivery technology, which uses CRISPR-Cas9 to insert CAR DNA to TRAC locus specifically in human T cells, produces moderate CAR-T cells compared with conventional ones. Earlier results of SC-gtCAR19, an anti-CD19 gtCAR-T candidate from an investigator initiated Phase I clinical trial (ChiCTR2000031942) demonstrated a favorable benefit-risk profile in relapsed/refractory B-Cell Acute Lymphoblastic Leukemia (r/r B-ALL) patients. Reported here are updated long-term follow-up results.
Objective The primary objective is to assess safety. Secondary objective is to evaluate efficacy. Level of CAR-T cell in blood was explored.
Method This is a single center, open-label and single-arm trial. Patients or allogeneic donors underwent leukapheresis and their CD3+ T cells were selected and modified by non-viral vector to produce SC-gtCAR19. A standard lymphodepletion chemotherapy was performed. The median dose was 3.89×106CAR+ cells/kg (0.58-8.31×106/kg). AEs were graded according to CTCAE v5.0. Cytokine Release Syndrome (CRS) and neurological events (NEs) were graded by ASTCT consensus grading. Relapse defined as bone marrow (BM) blast or B-ALL related mutation gene positive from nonexistence regardless of specific value, and extramedullary disease (EMD) occurrence.
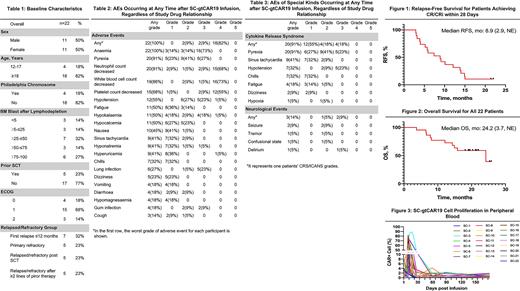
Result As of May 1st 2022, twenty-two r/r B-ALL patients received SC-gtCAR19 (Table 1). The median age was 29 (13-61), and the median number of previous regimen was 5 (2-16). The median baseline BM blast after preconditioning was 44.1% (0.3-99%), particularly 73% of patents with high disease burden (>25% leukemic blast). Five patients (23%) once had stem cell transplantation (SCT), and 14 (64%) have B-ALL related chromosomal and molecular abnormalities. One patient has EMD. No patient has been treated by any other CD19 related therapy before enrollment.
The most common AEs of any grade were anaemia (100%), pyrexia (91%), neutrophil count decreased (91%), and white blood cell count decreased (86%) (Table 2). CRS occurred in 20 patients (91%), and 4 of them (18%) with grade 3 CRS. The median time to CRS onset was 7 days (IQR 7-14), and the median duration of any grade CRS was 3 days (IQR 2-5). Three patents developed NEs, one was grade 2 and 2 were grade 3 (temporary seizure, related to infection and patient's cerebral infarction before enrollment). No fatal or life-threatening events happened. No Tocilizumab administered, four patients used vasopressors and 2 steroids for treatment of CRS.
All patients were eligible for efficacy analysis. At Day 28, the overall response rate was 100%, with 9 (41%) patients achieving complete remission (CR), ten (45%) achieving complete remission with incomplete haematological recovery (CRi), and 3 (14%) with blast-free hypoplastic. Among them, twenty patients (91%) achieved minimum residual disease (MRD) negative CR, and those 2 patients with MRD positive CR were assessed undetectable BM MRD in Day 90. Patient with EMD, the nodal lesions in her breast disappeared at Day 60.
At a median follow-up of 19.9 months (4.5-26.6), nineteen patients relapsed, within which 7 (36.8%) antigen escape relapse. The median duration of remission for 19 patients achieving CR/CRi was 8 months (2.5-NE). All patients received a consolidation treatment by Lenalidomide 3 months after infusion on condition of CR status until disease progression/death, and all of them did not receive SCT afterwards before relapse. As of the data cutoff, two patients (9%) had ongoing CR with median follow-up of 22.4 months. The median relapse-free survival for 19 patients was 8.9 months (2.9-NE) (Figure 1). The median OS was 24.2 months (3.7-NE) (Figure 2).
CAR T cell level measured by cell copies percentage per peripheral blood mononuclear cells peaked at median 12 days (IQR 10-13) (Figure 3). The median peak percentage of copies was 53% (IQR 26.5-72.1%).
Conclusion This study is the first clinical trial applied a gtCAR-T candidate for r/r B-ALL treatment. SC-gtCAR19 has a better risk-tolerance. Despite TCR gene knocked-out, gtCAR-T cell proliferation and its function both short and long term may not be influenced. We illustrate that this novel approach is feasible in terms of manufacture and clinical application.
Disclosures
Li:Sunnycell Therapeutics Ltd.: Current Employment, Current equity holder in private company. Peng:Sunnycell Therapeutics Ltd.: Current Employment, Current equity holder in private company.
OffLabel Disclosure:
Lenalidomide, for consolidation treatment 3 months after study drug infusion.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal